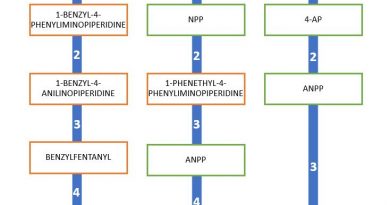
WHO: World Health Organization recommends five NPS for scheduling
Vienna, Austria – December 2023: Since its establishment in 2013, the UNODC Early Warning Advisory (EWA) on new psychoactive substances (NPS) regularly provides scientific information to Member States and the World Health Organization (WHO) for the identification of the most harmful, prevalent, and persistent NPS in support of review processes for national and international control. The WHO has announced the scheduling recommendations on psychoactive substances reviewed at the 46th Expert Committee on Drug Dependence (ECDD) held from 16-20th October 2023. In total, six substances were critically reviewed by the ECDD, five of which (one synthetic opioid, two stimulants, one dissociative and one sedative/hypnotic) were recommended for scheduling as listed below, while one benzodiazepine, flubromazepam,as well as nitrous oxide, a substance with medical and industrial use, were recommended to be kept under surveillance. The Committee also recommended that carisoprodol, a substance with medical uses, be subject to a future critical review.
Single Convention on Narcotic Drugs of 1961, as amended by the 1972 Protocol (Schedule I):
Convention on Psychotropic Substances of 1971 (Schedule II):
3-chloromethcathinone(3-CMC)
Convention on Psychotropic Substances of 1971 (Schedule IV):

Butonitazene, a synthetic opioid, was first reported in 2021 and belongs to the structural group of nitazenes. So far, 10 countries and territories have reported butonitazene to the UNODC EWA. The stimulants 3-chloromethcathinone and dipentylone are synthetic cathinones, which have been reported since 2015 and 2014 by 41 and 28 countries and territories respectively. The dissociative 2-fluorodeschloroketamine, a substance from the phencyclidine-type substances group, was first reported in 2016 and has since been reported by 39 countries and territories. The sedative/hypnotic bromazolam, a benzodiazepine, has been reported since 2016 by 29 countries and territories to the UNODC EWA.
Information on the identification of the five substances recommended for international control in forensic drug testing laboratories, is available on the respective substance pages (see links above) under – Analytical Information.
For more information, please see: